Dengue Virus (DENV) Antiviral Services
Antiviral assays against Dengue virus are performed to determine the inhibitory activity of small-molecule compounds and neutralizing antibodies for vaccine design. In vitro replication and infectivity assays against DENV-2 (16681 and TSV01) are routinely performed in monolayers of Vero and C6/36 cells. Several readouts are used to monitor virus replication, including specific ELISA assays and flow cytometry determination of intracellular viral antigens (e.g. capsid protein). These assays allow rapid and sensitive detection of Dengue virus replication in human and mosquito cell lines. Antiviral assays against other DENV strains are under development.
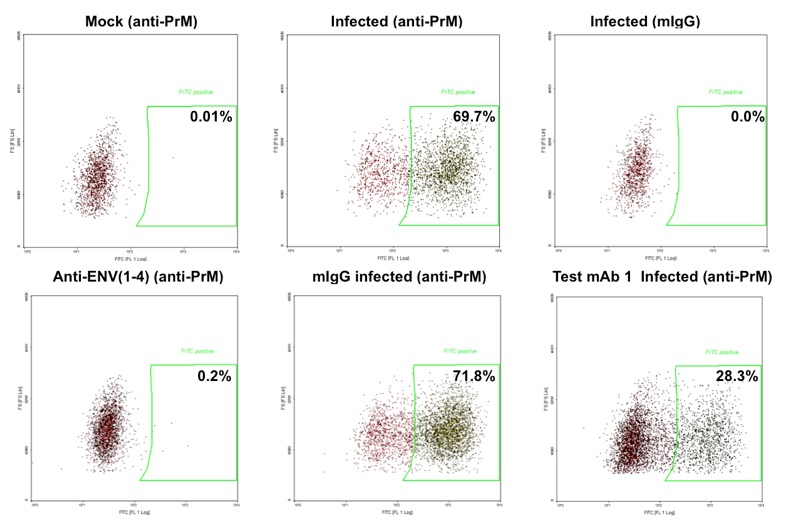
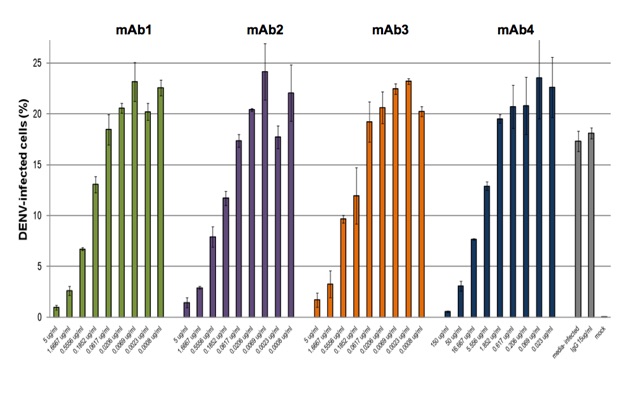
Flow-cytometry based assay to determine inhibition of DENV replication or neutralization with antibodies. In the above example incubation with test antibody 1 results in 60% inhibition of DENV infection. In the figure below dose-response studies with 4 mAbs neutralizing DENV-2 are shown. The assay is optimized and validated with DENV serotypes 1-4.
For additional information about Dengue antiviral services call us at (858) 677-9315 or contact us at antivirals@retrovirox.com
To find out about other antiviral assays offered at RetroVirox click on the following links:
Human Respiratory Syncytial Virus (HRSV)
Human Rhinovirus (HRV)
Hepatitis B Virus
Arenaviruses