Respiratory Syncytial Virus (HRSV) Antiviral Services
Our standard HRSV antiviral assays are performed in a number of permissive cells (Vero and Hep-2) infected with the strain Long or A2. Replication and neutralization of viral entry is evaluated by monitoring the production of viral antigens after staining with a cocktail of proprietary mAbs. This assay is amenable for HTS (validated on 96w format).
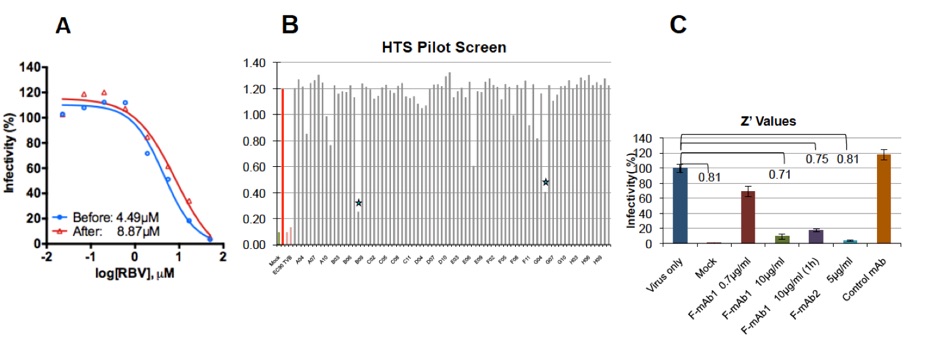
Validation of HRSV assay for HT screening of small-molecules (above). In (A) HEp-2 cells were infected with HRSV and the dose-response to ribavirin was determined after adding the inhibitor to the virus mixture or after cells had been incubated for 1h with virus. EC50 values are shown. (B) shows one representative plate of a pilot screen with 800 molecules to identify HRSV inhibitors. On the left, controls with vehicle alone, ribavirin 50μM and anti-F mAb (10 μg/ml) are shown. Two hits blocking more than 60% are marked with asterisks. (C) Displays the Z’ values obtained with the controls used in the HTS for HRSV inhibitors. Z’ values were obtained by comparing the positive signal with that of uninfected cells (mock) or cells treated with two neutralizing antibodies against F (1 and 2) added before or 1h after viral adsorption. Cells treated with control mAb are shown.
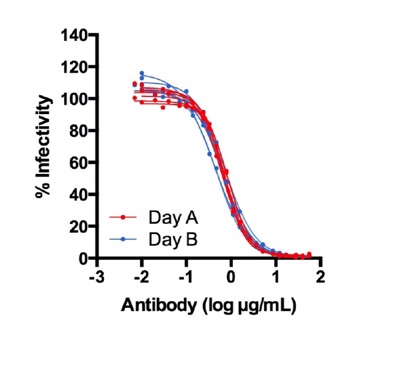
Inter-day variation of the assay. Left figure shows a second experiment where five identical dilutions of the test-item were processed in two different experiments separated by three days. EC50 values were derived with the GraphPad prism software dose response 4-parameter (4pl) fit curve.
For additional information about RSV antiviral services call us at (858) 677-9315 or contact us at antivirals@retrovirox.com
To find out more about other antiviral assays click on the following links:
Human Rhinovirus (HRV)
Hepatitis B Virus
Arenaviruses