Influenza Panel for Drug Susceptibility Testing
Influenza antiviral services at RetroVirox includes a panel of 20 influenza strains from multiple seasons for the evaluation of drug candidates, monoclonal antibodies, and antisera from animals and patients inoculated with candidate vaccines.
The component strains of the panel are changed from time to time to incorporate more recent strains. As of May 2019 the panel is comprised of 20 influenza A strains (table below) from seasons spanning from 1933 to 2012, and includes strains recommended by WHO for the production of vaccines in past years, such as A/California/2009, A/Brisbane/2007, A/Solomon/2006 and A/New York/2004. Several pandemic strains from the 2009 and 2012 seasons are also included. This panel will constitute an invaluable tool to determine the breadth and potency of your candidate drugs and antibodies. It can also be used to determine the level of protection of antisera generated with candidate vaccines tested in animal models or humans.
The assay utilizes infected A549 cells (human lung epithelial carcinoma cells) to evaluate the ability of test-items to block influenza replication or viral entry. The test detects the presence of intracellular viral antigens in influenza-infected cells. Antigen immunodetection is performed with a proprietary cocktail of antibodies designed for sensitive detection of multiple influenza A strains. The assay is fully optimized with 20 different strains, and it has been validated with neutralizing antibodies and known inhibitors of influenza replication (e.g. baloxavir, ribavirin and oseltamivir). Upon request, additional strains can be incorporated in the panel. The assay is performed in a 96-well format and up to 20 test-items can be evaluated per month with the full panel (providing full dose response curves and EC50 values). This is an assay recommended for drug candidates whose anti-influenza activity has been previously confirmed. For non-validated drug candidates or early hits, we recommend the use of our standard anti-influenza assay performed with the strain A/PR/8/1934.
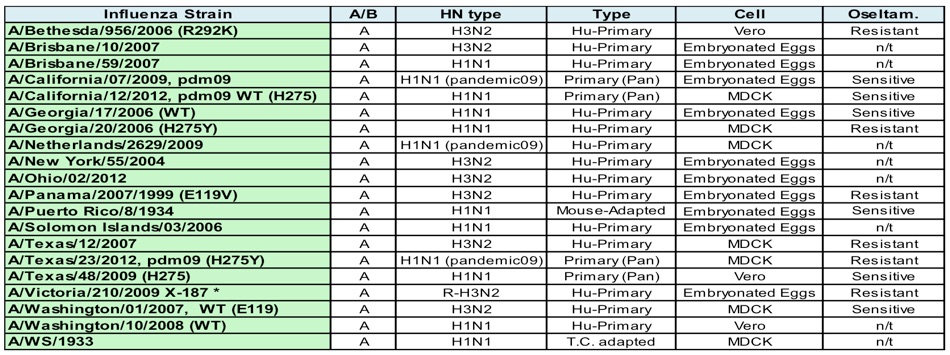
Influenza A Strain Panel. Twenty Influenza A strains are available to test drug susceptibility. The H/N serotype, source, and sensitivity to oseltamivir with the different influenza inhibitors is shown. Other viruses may be available upon request. *A/Victoria/2009/H3N2 is a reassortant known as A/NYMC X-187 (Victoria/210/2009 x Puerto Rico/8/1934) (H3N2)
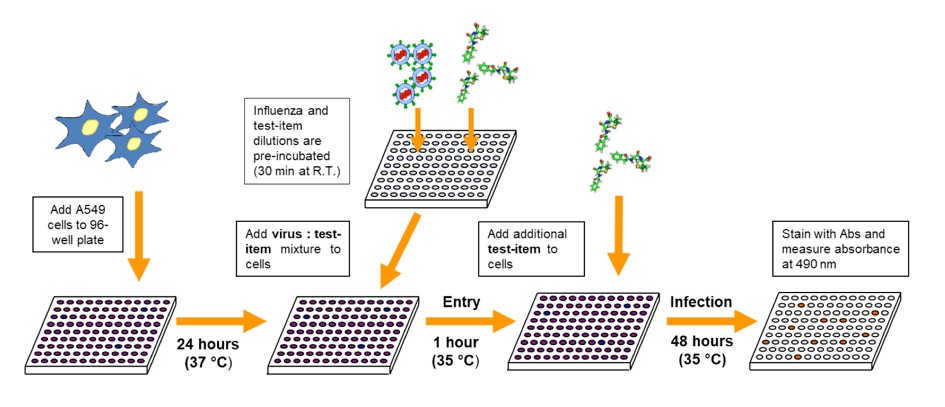
Experimental outline utilized in the Influenza Panel Assay. Cells are seeded 24 hours prior to infection. Then, pre-incubated mixtures of influenza and test-items are added to the cells for 1 hour at 35°C to allow viral entry. After adsorption, additional test-item is added to the infection plate and infection is allowed for 48 hours. After that period, cells are fixed, stained with a cocktail of mouse monoclonal antibodies, and the amount of viral antigen present is revealed with a colorimetric reaction. Absorbance at 490 nm is monitored to determine the level of influenza antigens present in the cells. Note that this assay includes a pre-incubation of virus with test-item and therefore can reveal the activity of both, inhibitors of entry, and inhibitors of influenza replication.
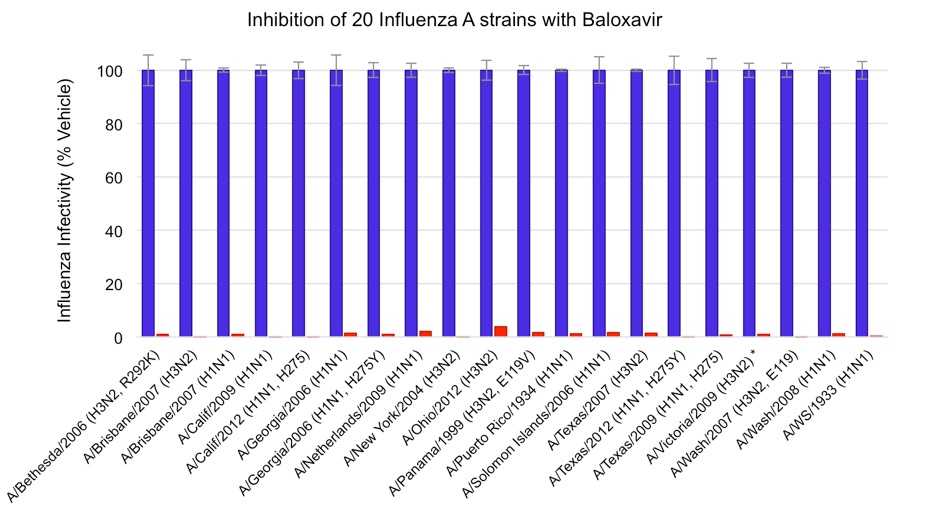
Replication of 20 influenza virus strains in A549 cells in the presence or absence of antiviral Baloxavir. The extent of infection is indicated as a percentage of vehicle control. Raw OD values from mock-infected cells were subtracted from all data points. Standard deviation of duplicate vehicles from each influenza A strain are shown.
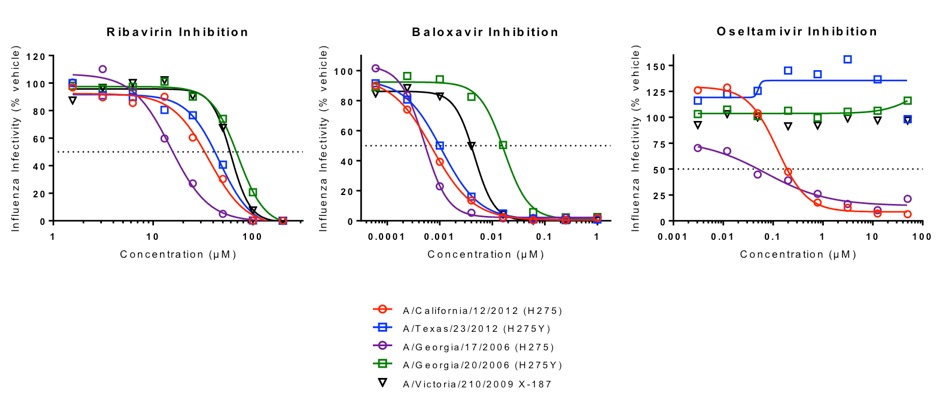
Inhibition of five representative Influenza A strains. Infections in the presence of ribavirin, baloxavir, or oseltamivir were monitored in A549 cells infected with five representative Influenza A strains. The extent of virus replication is indicated as a percentage of vehicle control. Data was adjusted to a sigmoidal function in GraphPad Prism.
For additional information about the influenza panel please call us at (858) 232-7919 or contact us at antivirals@retrovirox.com
To see more information about other antiviral assays offered against other viruses click on the following links:
Human Respiratory Syncytial Virus (HRSV)
HIV Pseudoviruses for Neutralization Assays