Pseudoviruses for Evaluation of Entry Inhibition with Highly Pathogenic Viruses
Pseudotyping HIV virus with heterologous viral envelope proteins can be used as a surrogate assay to evaluate viral entry against highly pathogenic viruses, which otherwise must be handled in Biosafety Level 4 facilities. The use of HIV pseudoviruses allows to evaluate inhibition of viral entry under Biosafety Level 2 facilities.
Assays with HIV pseudoviruses are available for two hemorrhagic fever (HF) viruses: Lassa fever virus (LASV), and Ebola virus (EBOV). Assays are also available for the evaluation of entry inhibitors against several Avian Influenza pandemic viruses, and several human coronaviruses (CoV), including SARS-CoV-2 (COVID-19) and SARS-CoV. These assays allow us to evaluate the neutralization activity of monoclonal antibodies, antisera and small-molecule entry inhibitors against LASV, EBOV, Ebola, SARS-CoV-2, SARS-CoV, and avian influenza viruses. Pseudoviruses carrying the envelope glycoproteins of HIV or VSV (vesicular stomatitis virus) can be used as controls to determine the specificity of your entry inhibitors.
Additional pseudovirus against other pathogenic viruses can be developed and validated upon request.
In our facilities we can perform high throughput screening (HTS) of small-molecule libraries (ranging from 10,000 up to 100,000 molecules) and antibody libraries using HIV pseudoviruses carrying heterologous envelope proteins from HF and avian influenza viruses. Additional information about our HTS capabilities is available here.
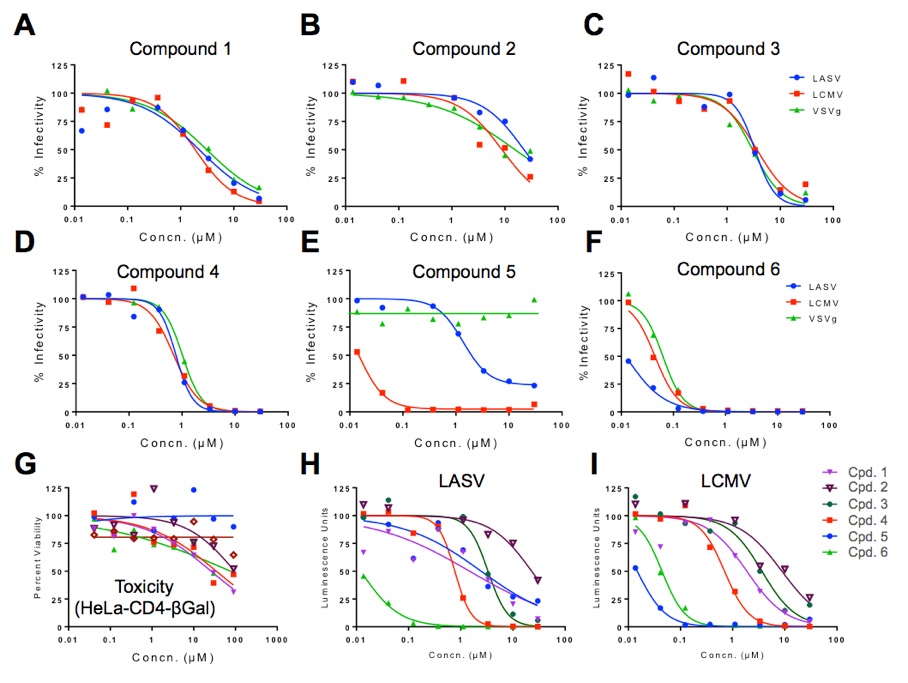
Evaluation of Inhibitors Entry of Lassa Fever Virus. Six different compounds were evaluated for inhibition against the highly pathogenic Lassa fever virus (LASV) utilizing HIV pseudoviruses carrying the LASV envelope protein. As controls for specificity, HIV pseudoviruses with the envelope from a related arenavirus (LCMV) or with the glycoprotein of the distant vesicular stomatitis virus (VSV), were studied in parallel. Compound 5 (figure E) displayed activity against the Old World arenaviruses Lassa and LCMV, but showed no inhibition against HIV carrying VSVg, and displayed no toxicity against the cells used in the assay (figure G). However, compound 1- 4 and 6 blocked all the pseudoviruses (and also HIV infection, not shown). These findings suggest that compound 5 is an entry inhibitor of Old World arenaviruses, whereas the remaining compound 6 inhibits infection at later stages. The pseudovirus assay was later used to monitor the optimization of the activity of comopund 5.
For additional information about the use of HIV pseudovirus to evaluate entry inhibitors of highly pathogenic viruses call us at (858) 232-7919 or contact us at antivirals@retrovirox.com
To find out about other antiviral assays offered at RetroVirox click on the following links:
Human Respiratory Syncytial Virus (HRSV)
HIV Pseudoviruses for Neutralization Assays